
Studies with Liv.52 in the Treatment of Infective Hepatitis, Chronic Active Hepatitis and Cirrhosis of the Liver
Prof. Mandal, J.N., M.B. (Cal.), D.C.H. (Lond.), F.R.C.P. (Edin.), Professor - Director
and Roy, B.K., M.D. (Cal.), Senior House Physician to Professor-Director, Department of Medicine, Medical College and Hospital, Calcutta, India.
Liv.52 in chronic active hepatitis and cirrhosis
Table XXII shows the Mean values and the trend of changes in the Total protein, Albumin and Globulin in the two groups of cases.
(v) SGPT / SGPT: Serum GOT and specially GPT were found to be high in almost all cases of cirrhosis of the liver in the series. (1) As in cases of other biochemical profiles, Serum GOT as well as GPT levels in 4 cases on Liv.52 became normal in 12 months and remained normal for the next 24 months; no case in the Control Group showed such improvement in 12 months. (2) In 10 cases on Liv.52 Serum GOT and GPT levels became normal to almost normal in 18-24 months and remained normal in subsequent 12 months as against 3 cases (3/8) of the Control Group showing slight improvement during that period. (3) Five cases on Liv.52 (5/19) and 5 cases in the Control Group (5/8) failed to show any improvement in their SGOT and SGPT levels, rather they gradually worsened, even after treatment for 36 months.
Table XXIII shows the Mean values and the trend of changes in Serum GOT and GPT in Cirrhosis of the liver in both in the Liv.52 Group and the Control Group.
(vi) BSP Studies: BSP excretion studies could be performed on only 5 cases on Liv.52 and 5 cases of the Control Group. Four cases on Liv.52 (4/5) showed greater excretion of BSP in 12-18 months as against only one case of the Control Group.
(vii) Protein Electrophoresis: Increased level of all fractions of immunoglobulin, particularly IgG, was found in all cases of Cirrhosis patients before treatment.
IgG became normal in 14 cases on Liv.52 after 18-24 months. In no case was there any improvement in the IgG level in the all cases of Cirrhosis patients before treatment.
IgG became normal in 14 cases on Liv.52 after 18-24 months. In no case was there any improvement in the IgG level in the Control Group. In fact, in 5 cases it rose to a higher level.
| Table XXII: Serum total protein, Albumin : Globulin in cirrhosis of the liver (in gms/100 ml) | ||||||||||||||||||||||||
| On admission | 3 months | 6 months | 9 months | |||||||||||||||||||||
| TP | AL | GL | TP | AL | GL | TP | AL | GL | TP | AL | GL | |||||||||||||
| 1 | 2 | 3 | 4 | |||||||||||||||||||||
| Liv.52 group (Mean: 19 cases) | 6.2 | 2.3 | 3.9 | 6.3 | 2.5 | 3.8 | 6.4 | 2.7 | 3.7 | 6.5 | 3.0 | 3.5 | ||||||||||||
| Control group (Mean: 8 cases) | 5.6 | 1.8 | 3.8 | 5.7 | 1.7 | 4.0 | 6.0 | 1.8 | 4.2 | 6.1 | 1.9 | 4.2 | ||||||||||||
| 12 months | 15 months | 18 months | 21 months | 24 months | ||||||||||
| TP | AL | GL | TP | AL | GL | TP | AL | GL | TP | AL | GL | TP | AL | GL |
| 5 | 6 | 7 | 8 | 9 | ||||||||||
| 6.6 | 3.1 | 3.5 | 6.6 | 2.9 | 3.7 | 6.7 | 3.2 | 3.5 | 6.8 | 3.5 | 3.3 | 6.8 | 3.7 | 3.1 |
| 6.2 | 2.1 | 4.1 | 6.3 | 2.2 | 4.1 | 6.4 | 2.3 | 4.1 | 6.6 | 2.4 | 4.2 | 6.8 | 2.4 | 4.4 |
(v) SGPT / SGPT: Serum GOT and specially GPT were found to be high in almost all cases of cirrhosis of the liver in the series. (1) As in cases of other biochemical profiles, Serum GOT as well as GPT levels in 4 cases on Liv.52 became normal in 12 months and remained normal for the next 24 months; no case in the Control Group showed such improvement in 12 months. (2) In 10 cases on Liv.52 Serum GOT and GPT levels became normal to almost normal in 18-24 months and remained normal in subsequent 12 months as against 3 cases (3/8) of the Control Group showing slight improvement during that period. (3) Five cases on Liv.52 (5/19) and 5 cases in the Control Group (5/8) failed to show any improvement in their SGOT and SGPT levels, rather they gradually worsened, even after treatment for 36 months.
Table XXIII shows the Mean values and the trend of changes in Serum GOT and GPT in Cirrhosis of the liver in both in the Liv.52 Group and the Control Group.
| Table XXIII: Serum SGOT and SGPT in cirrhosis of the liver (in Karmen units) | ||||||||||||||||
| On admn. | 3rd month | 6th month | 9th month | |||||||||||||
| GOT | GPT | GOT | GPT | GOT | GPT | GOT | GPT | |||||||||
| 1 | 2 | 3 | 4 | |||||||||||||
| Liv.52 group (Mean: 19 cases) | 21.1 | 47.7 | 21.9 | 48.4 | 21.0 | 47.0 | 19.9 | 45.4 | ||||||||
| Control group (Mean: 8 cases) | 31.2 | 53.7 | 23.2 | 57.8 | 25.2 | 67.0 | 26.0 | 63.6 | ||||||||
| 12th month | 15th month | 18th month | 21st month | 24th month | |||||
| GOT | GPT | GOT | GPT | GOT | GPT | GOT | GPT | GOT | GPT |
| 5 | 6 | 7 | 8 | 9 | |||||
| 18.3 | 43.3 | 17.8 | 43.1 | 21.6 | 53.0 | 19.2 | 48.1 | 15.8 | 40.9 |
| 37.1 | 69.8 | 29.6 | 72.1 | 28.7 | 72.8 | 32.2 | 71.8 | 31.2 | 69.8 |
(vi) BSP Studies: BSP excretion studies could be performed on only 5 cases on Liv.52 and 5 cases of the Control Group. Four cases on Liv.52 (4/5) showed greater excretion of BSP in 12-18 months as against only one case of the Control Group.
(vii) Protein Electrophoresis: Increased level of all fractions of immunoglobulin, particularly IgG, was found in all cases of Cirrhosis patients before treatment.
IgG became normal in 14 cases on Liv.52 after 18-24 months. In no case was there any improvement in the IgG level in the all cases of Cirrhosis patients before treatment.
IgG became normal in 14 cases on Liv.52 after 18-24 months. In no case was there any improvement in the IgG level in the Control Group. In fact, in 5 cases it rose to a higher level.
| (viii) Histopathological Examination: (1) Four cases on Liv.52 showed remarkable improvement in 12 months the picture showing almost normal liver structure with minimal fibrosis, and remained so until 24 months later. None of the Control Group revealed any remarkable changes in histopathology from that in the initial stage (even after 9-12 months’ treatment). (Annexure VII, VIII and IX). (2) Ten cases on Liv.52 (10/19) showed moderate improvement in the liver architecture in 18 to 24 months and the fibrosis did not progress in subsequent 12 months’ follow-up, whereas 3 cases in Control Group (3/8) showed early similar improvement in 24 months, but the fibrosis was more pronounced, when examined 12 months later. (3) Five cases on Liv.52 (5/19) who had marked destruction of the liver architecture with pronounced fibrosis showed no improvement, even after 36 months of intensive therapy. Five cases of the Control Group (5/8) behaved in a similar manner, rather the histopathological picture deteriorated further at the end of the study. | 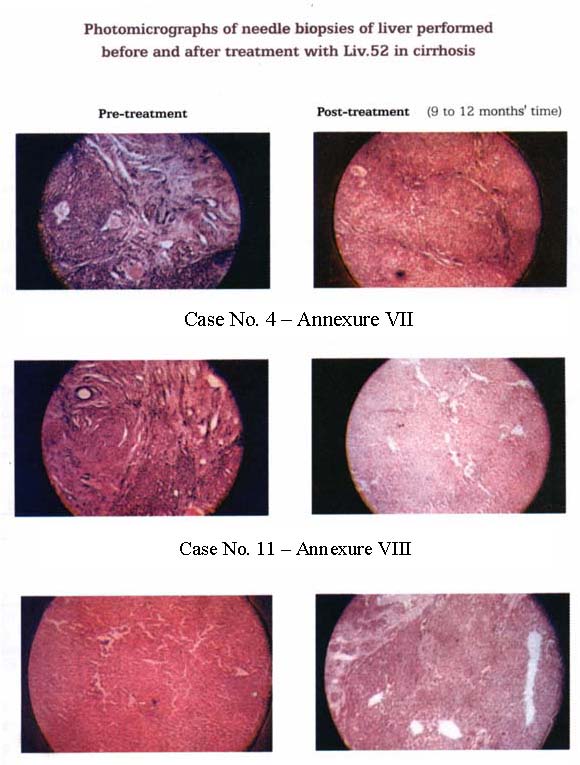 |
Analysing the 27 cases of Cirrhosis of the Liver 19 belonging to Liv.52 Group and 8 belonging to Control Group clinically, biochemically and histopathologically, the following inferences may be drawn as regards results, presented in Table XXIV.
| Table XXIV: Results of treatment in cirrhosis of the liver | |||||||||||
| No. of cases | |||||||||||
| Liv.52 group (19 cases) | Control group (8 cases) | ||||||||||
| (a) | Good | 4 (21.05%) | Nil (0%) | ||||||||
| (b) | Fair | 10 (52.63%) | 3 (37.50%) | ||||||||
| (c) | Poor | 5 (26.32%) | 5 (62.50%) | ||||||||
| Criteria for Results: 'Good', 'Fair' and 'Poor' in Chronic active hepatitis and Cirrhosis of the liver. | |||||||||||
Good: Improved clinically, biochemically and histopathologically in 6-12 months and remained so up to subsequent 36th month of follow-up.
Fair: Improved clinically, biochemically and histopathologically in 18 to 24 months and remained so up to 36th month as follow-up.
Poor: No improvement/deterioration in 24 to 36 months.
DISCUSSION
Hepatic damage in any form is a difficult condition to treat. Though the liver has great reserves and a good power of regeneration, it frequently happens that if and when the initial insult in severe and/or the pathological process is prolonged, permanent and irreversible, changes may occur undermining the capability of the organ to maintain its multifarious normal functions, thereby leading to a significant morbidity and often mortality affecting the unfortunate victims. No “Specific” treatment has so far been evolved for the treatment of the spectrum of cases selected for the present study, viz., Viral Hepatitis, Chronic Active hepatitis and Cirrhosis of the Liver, and the different regimes of treatment advocated have remained mostly symptomatic and at the most palliative as has been expressed earlier in the introductory comments. It would appear imperative, therefore, for every clinician and research worker to look for a drug that would help these difficult and trying maladies, or at least minimise the ravages and complications associated with or precipitated by them.
Keeping the above basic facts in mind it would appear from the results of the present study that the indigenous preparation Liv.52, though not a “Specific” remedy, has a definite beneficial effect on the foregoing conditions, much more than what could be achieved by other conventional therapies tried so far. The study also provides a good corroboration of the earlier findings of other workers in the field (loc. cit.) who have tried the preparation over a number of years with highly encouraging results.
The efficacy of the drug may now be highlighted category-wise in the three conditions where it was tried in the present study:
(1) Infective Hepatitis: Though in itself this is mostly a self-limiting disease resolving itself within a few weeks, experience in India reveals that the disease tends to assume a more severe form with a rather prolonged course with a attendant greater propensity for serious complications than encountered in other advanced countries as pointed out earlier. Therefore, the aim in the treatment should be to cut short the course and prevent the complications of the disease as far as possible. This target appears to have been achieved in the present study corroborating the findings of other workers.
(a) Clinical: Majority of the cases treated with Liv.52 had significantly earlier resolution of the symptomatology, viz., fever, anorexia, nausea, epigastric pain, jaundice, hepatomegaly etc. than in the Control Group. The findings in the present study compare well with the findings of Sule et al. (1965)1, Ramalingam et al. (1971)2, Mukherjee et al. (1970)6, Patel et al. (1972)10 and Mehrotra et al. (1973)11 and others all of whom have reported uniformly good and earlier resolution of the symptomatology in majority of their cases.
(b) Biochemical (Liver Function Tests): (i) Serum Bilirubin: In the present study, 73.33% of cases had an earlier clearance of Serum bilirubin (within 2-3 weeks) than in the Control Group. The corresponding finding in the literature reports are: Sule et al.1 – 76%, Ramalingam et al.2 – 80%, Patel et al.10 – 74% and Mehrotra et al.11 – 77.14%. The high percentage of cases showing significantly earlier clearance of Serum bilirubin in these cases may encourage one to use the preparation specially in cases with cholestasis in preference to steroids, in consideration of the comparative safety of the former as against the possible hazards of the latter. Steroids, however, were not used in any of the cases under study.
(ii) Alkaline Phosphatase: As observed in respect of Serum bilirubin, this elevated serum enzyme also showed an early clearance in 73.33% as against only 26.66% of the Control Group. Dave et al (1972)3 made similar observations in 68.2% of his cases, Sule et al.1 in 71.5% and Patel et al.10 in 63%. Gupta et al. (1972)4 however reported no significant clearance of the enzyme in a limited number of cases in his study.
(iii) SGOT and SGPT showed earlier clearance in the Liv.52 Group in 73.33% of cases comparing favourably with the findings of Sule et al.1: 78.4%, Patel et al,10: 77.8%, Gupta et al,4 also reported similar findings.
(iv) Serum Total Protein / Albumin: Globulin showed significant earlier improvement in their values in the Liv.52 treated cases but references were inadequate for comparison.

Copyrights © 2009 healthyliver.co.uk
